BIA-ALCL Disease Summary
Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) is a rare and emerging disease that has been found to develop around breast implants that have a rough textured surface. The US FDA has posted safety updates in 2011, 2016, and 2017 warning that women with textured breast implants have a low but increased risk of developing anaplastic large cell lymphoma. This is a form of lymphoma and is a cancer of the immune system. This is not a type of breast cancer. The main symptom of BIA-ALCL is an increase in breast size from fluid collecting more than one year after placement of an implant, and most often eight to ten years after implant placement. Some patients may see lumps in the breast or armpit or hardening of the breast.
When caught early, BIA-ALCL is usually curable in most patients. The risk of developing BIA-ALCL is between 1 in 1000 to 1 in 30,000 women with textured breast implants. BIA-ALCL has been found with both silicone and saline implants and both breast cancer reconstruction and cosmetic patients. There have been no confirmed cases of BIA-ALCL in patients with a history of only smooth implants. BIA-ALCL patients seem to have an allergy reaction to textured breast implants over many years. At this time, it is not possible to test for who is at risk of this disease. Any patient experiencing these or any symptoms should see their doctor for evaluation.
For Women Considering Implant Reconstruction
Breast cancer patients considering implant reconstruction should discuss the benefits and risks of different types of implants with their doctor. There are many breast implant options such as smooth, textured, round, shaped, saline, and both liquid and solid silicone. A physician may suggest a certain implant shape, surface, and fill to achieve an optimal reconstruction while minimizing potential complications. BIA-ALCL is an emerging risk of textured implants and patients should be aware when choosing the implant that is right for them.
For Women Who Have Textured Implants
The World Health Organization first classified BIA-ALCL as a new disease in 2016. This has created more awareness and education about the disease. Women who already have textured implants have been understandably concerned about BIA-ALCL. Some patients without symptoms may want to be tested for BIA-ALCL “just in case.” At this time it is not possible to test a patient without the symptoms as the test is performed on the large fluid buildup around an implant. The FDA does not suggest additional screening of patients without symptoms.
Routine breast health includes self breast examinations, physician checkups, and mammograms. A small or minimal amount of fluid around an implant may be routinely found on imaging tests. This minimal fluid is normal and does not need to be tested for BIA-ALCL. Women without symptoms or diagnosis BIA-ALCL should not have their implants removed or exchanged “just in case”, as the risks of any surgery is still greater than the risk of developing BIA-ALCL. Women with textured implants should not be alarmed or panic, but should be informed and aware of BIA-ALCL so they can see their physician if they find any unusual symptoms.
For Women who develop symptoms of BIA-ALCL
The symptoms of BIA-ALCL include breast enlargement, asymmetry, or a large fluid collection developing more than one year after receiving an implant.
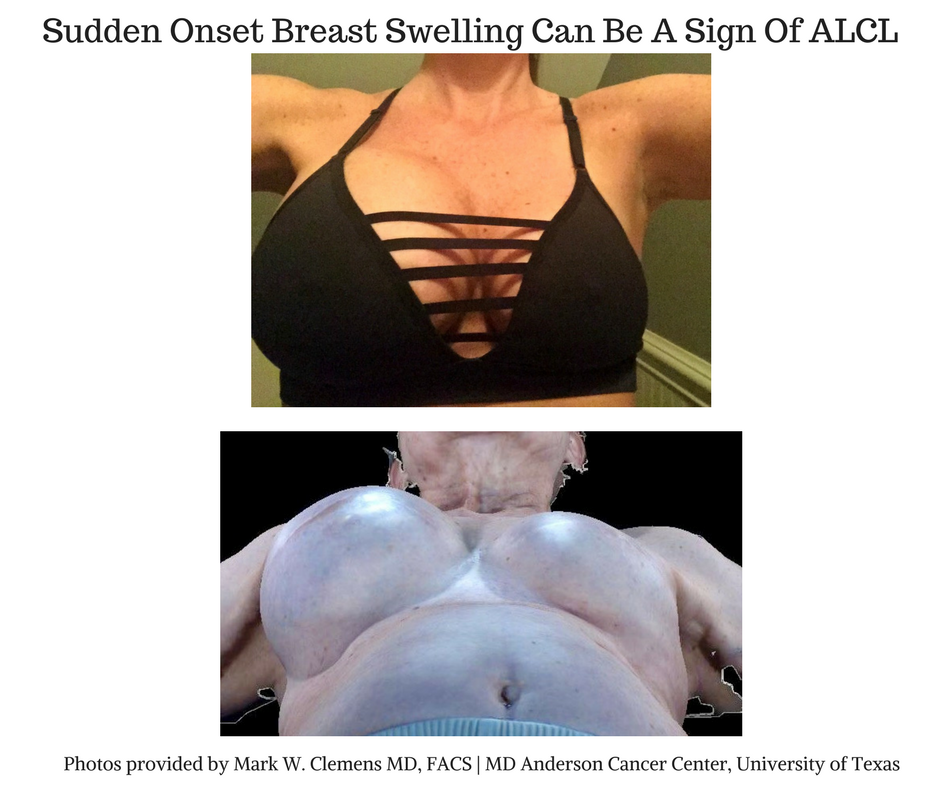
BIA-ALCL patients most commonly developed the disease eight to ten years after having a textured breast implant placed. Some patients may have a lump in the breast or armpit. Some patients developed an overlying skin rash or hardening of the breast. Women who develop these symptoms should see their physician to be evaluated with a physical exam and may require further testing.
Patients may receive an ultrasound or a magnetic resonance imaging (MRI) of an enlarged breast to evaluate for fluid or lumps around the implant. If fluid or a mass is found, patients will require a needle biopsy to test for disease. A fine needle biopsy is performed in a clinic by their physician or by an interventional radiologist using ultrasound guidance. Needle biopsies do not require surgery. The biopsy draws fluid from around the implant. This fluid is then tested for CD30 immunohistochemistry (CD30 IHC) by a pathologist. This is the screening test for BIA-ALCL and may take a week for evaluation. CD30 IHC is a common test, can be performed in any hospital and pathology lab, and does not require a specialized center. Some community hospitals may send specimens to a larger academic hospital if the lab results are confusing, suspicious, or indeterminate. Testing for CD30 IHC is required to make a diagnosis or rule out BIA-ALCL and is covered by all health insurance programs because the test is an investigation of a possible cancer. Fluid collections around a breast may happen without BIA-ALCL and are called seromas. Once BIA-ALCL has been ruled out, a physician can treat a seroma as they normally would.
For Women Diagnosed with BIA-ALCL
Women hearing the diagnosis of BIA-ALCL may feel shocked, frightened, and anxious. Any diagnosis of cancer is understandably a very scary situation. BIA-ALCL patients should know that not all cancers are equal and when caught early, BIA-ALCL is curable in most patients. The National Comprehensive Cancer Network (NCCN) has set physician guidelines for the treatment of BIA-ALCL that use proven methods of treatment.
When a women is diagnosed with BIA-ALCL, her physician will ask for a PET/CT Scan. This scan is performed before any treatment or surgery. BIA-ALCL usually is located just around the breast implant. However, a PET/CT scan looks for any disease that may have spread throughout the body. Any spread of the disease tells the stage which is important for treatment. Lumps in the armpit may be disease that has spread to the lymph nodes or may still be a normal enlargement of the lymph nodes. Testing of the lymph nodes may be performed with a needle biopsy or with a surgery to remove a lymph node for testing. Additional tests may sometimes include blood tests and a bone marrow biopsy.
For patients with BIA-ALCL disease only around the implant, surgery is performed to remove the breast implant and the scar capsule around the implant. This is sometimes referred to as a “total capsulectomy” or less commonly as an “en bloc resection”. A larger surgery may be required to remove lumps that have formed around the breast implant or on the chest. Involved lymph nodes may require surgery for removal. Surgeries can be performed by a general surgeon, plastic surgeon, breast surgeon, or oncology surgeon. BIA-ALCL does not require a mastectomy as this is not a disease of the breast tissue. For most patients, surgery alone is able to completely treat BIA-ALCL when the disease is located around the breast implant.
The removal of a breast implant and capsule usually creates skin redundancy and a deflated look to the breast. Some patients have chosen no reconstruction. Some patients have chosen to have a breast lift (mastopexy) at time of implant removal to reshape the breast and remove excess skin. Some patients have chosen to use their own tissue for reconstruction such as fat grafting or tissue flaps (e.g. DIEP, TRAM, Latissimus Dorsi Muscle flap). Some patients have chosen reconstruction with a smooth surface implant. Patients should evaluate all of the reconstructive options and know that these some techniques can be done at time of surgery or delayed months to years based on the patient’s request.
Patients with more advanced forms of BIA-ALCL may have disease in their lymph nodes or in their bones and organs. These patients require further treatment with chemotherapy. Recommendations for chemotherapy are made by an oncologist with knowledge of lymphomas and NCCN guidelines. Chemotherapy may be one medication or a combination of several. Chemotherapy requires receiving intravenous medication several times a week for several months. Side effects and symptoms of chemotherapy may occur and should be discussed with an oncologist. Additional treatments for BIA-ALCL are rare such as radiation therapy and stem cell transplant, but may be recommended by your doctor in advanced cases.
After treatment for BIA-ALCL, patients are followed for two years with regular follow ups and imaging tests in three to six month intervals. Disease recurrence is rare and usually happens in the first year after treatment if it does occur.
At this time, the treatment of BIA-ALCL is covered by health insurance companies and specifically guaranteed from some providers. Reconstruction may or may not be covered for BIA-ALCL following cosmetic augmentation but is guaranteed for breast cancer reconstruction patients.
Finding a Surgeon for Testing and Treating of BIA-ALCL
Women with symptoms of BIA-ALCL should see their plastic surgeon for testing. Screening for BIA-ALCL includes a needle aspiration and testing for CD30 IHC. This test is common and does not require a specialized center for evaluation. Patients needing treatment when diagnosed with BIA-ALCL may seek physicians familiar with the disease. A cancer center team made up of surgical oncologist, plastic surgeon, pathologist, and lymphoma oncologist can help address the complexities of treatment of a rare disease.
Reporting of Confirmed Cases
The FDA specifically recommends that all confirmed cases be reported to the PROFILE registry. If you have been diagnosed with BIA-ALCL, please ensure your physician has reported the case to the PROFILE registry for tracking of cases.
Additional information on BIA-ALCL can be found here:
MD Anderson Cancer Center Patient Information on BIA-ALCL
FDA Updates on Breast Implants and BIA-ALCL
The PROFILE Registry from the Plastic Surgery Foundation and American Society of Plastic Surgeons
Australian Government Department of Health on BIA-ALCL
Content provided by Mark W. Clemens, MD FACS | MD Anderson Cancer Center